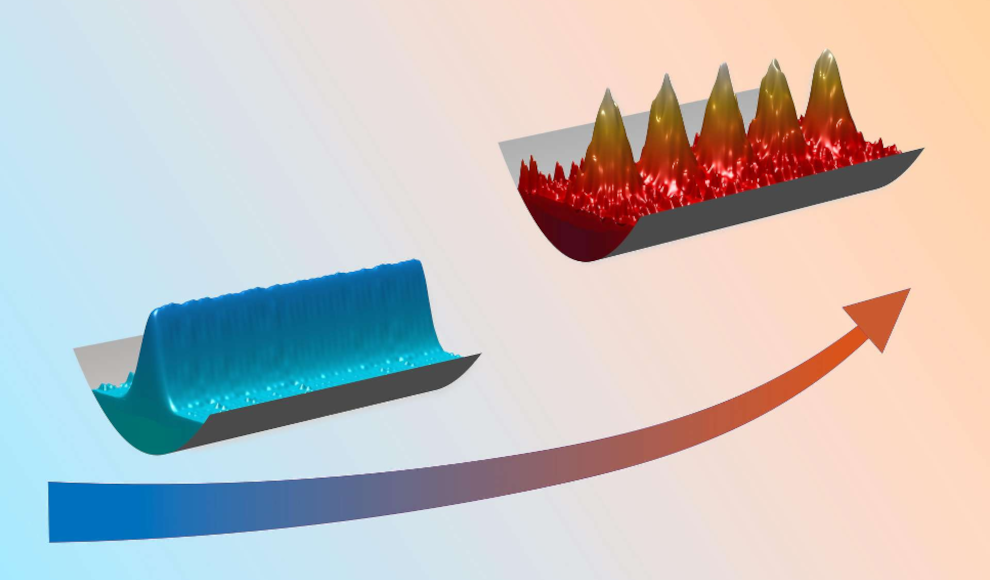
In the world of classical physics, heating a material causes its atoms to move thermally, resulting in the melting of a solid and eventually the evaporation of a liquid. However, scientists at Aarhus University have discovered that in the quantum world, this process can occur differently. According to their publication in the journal Nature Communications, a dipolar quantum liquid can become solid when heated, forming crystalline structures. The basis of their research is suprasolidity, a quantum mechanical state of matter that exhibits properties of both solid and superfluid bodies simultaneously.
Suprasolidity was first predicted in 1969 by David J. Thouless, Alexander Andrejew, and Ilja Lifschiz. In 2019, physicists at the University of Stuttgart experimentally demonstrated the theory of suprasolidity for the first time. In 2021, scientists at the University of Innsbruck were able to create a two-dimensional suprasolid body. They observed that quantum gases made up of strongly magnetic atoms at a temperature near absolute zero formed both a superfluid liquid and a crystal in parallel. Unlike partially melted ice, suprasolid bodies do not have solid and liquid components side by side. Instead, the two states of matter overlap in a quantum mechanical way, with their atoms simultaneously part of the superfluid and the crystal.
Now, researchers at Aarhus University, in collaboration with researchers at the University of Innsbruck, have solved the mystery of suprasolid structures. They analyzed the state of a gas made up of strongly magnetic dysprosium atoms and made an unexpected observation: an increase in temperature promotes the formation of suprasolid structures. Based on this discovery, the physicists developed a theoretical model that explains the experimental results. The behavior of the quantum liquid is due to the directionality (anisotropy) of the dipole-dipole interaction of the magnetic dysprosium atoms, which acts both attractively and repulsively in parallel. Additionally, the scientists were able to create a phase diagram that illustrates the formation of suprasolid states at different temperatures.