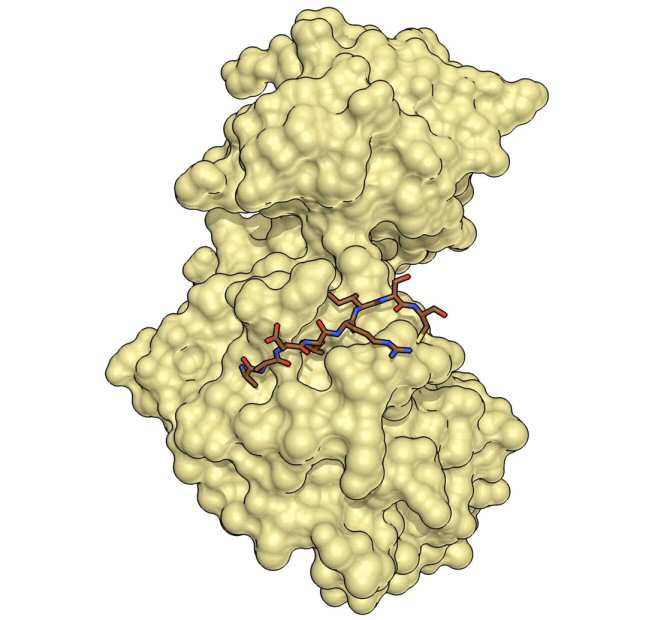
A team of scientists led by Dr. Maria Macias and Dr. Angel R. Nebreda, both ICREA researchers at the Institute for Research in Biomedicine in Barcelona (IRB Barcelona), has discovered a new form of the protein p38a. This protein is a key enzyme in the regulation of various cellular functions and plays a crucial role in diseases such as cancer, chronic inflammation, and neurodegenerative diseases. The researchers found that p38a adopts a previously unknown “oxidized” conformation, which it would transiently adopt depending on the redox state of the cell, in which a disulfide bridge is established. This new form of p38a, described in the journal Nature Communications, does not allow binding with activators or substrates, so it is unable to perform the characteristic functions of this kinase. This reversible process opens up new avenues for the development of therapeutic compounds that modulate the activity of p38a more precisely.
There are 357 published structures of the p38a protein in the Protein Data Bank database, but all correspond to its reduced form, the only one known until now. In the oxidized form described in this study, a disulfide bridge is established that forces a conformational change that blocks access to the activator and substrate binding site. This is a new inactive form of the p38a protein that would be present under certain cellular conditions. The study of kinases in their oxidized form is complex due to the influence of oxidative stress conditions and the transience of these forms in the cellular environment, but they may hold the key to effectively addressing them from a pharmacological point of view.
This new form of p38a illustrates a mechanism of action regulated by the cellular redox state, explaining biochemical observations described so far but without a structural molecular basis. In future work, the researchers will focus on exploring new interaction cavities that appear in the oxidized form, as they may help inactivate the protein without intervening in the catalytic center, thus gaining specificity. The study was carried out in collaboration with the laboratory of Dr. Modesto Orozco at IRB Barcelona and the University of Barcelona, and with Nostrum Biodiscovery. The work is titled “Structural basis of a redox-dependent conformational switch that regulates the stress kinase p38α” and has been published in the academic journal Nature Communications.