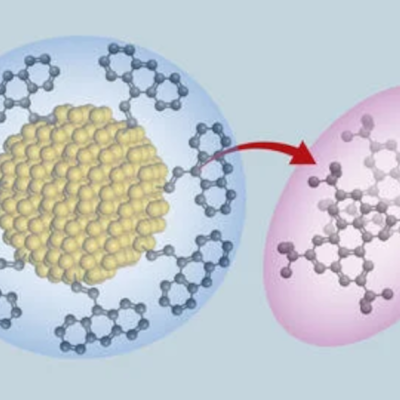
Clean water is a scarce resource in many parts of the world, and the challenges of climate change and population growth are making it even more difficult to access. Researchers at the University of California in Berkeley have developed a new membrane that simplifies the process of obtaining clean water from seawater. The traditional method involves desalinating the seawater and then removing heavy metals and other pollutants in several steps, which is both time-consuming and expensive. However, the new membrane, which contains nanoparticles, can filter out toxic substances and useful resources such as iron, gold, and copper from seawater.
The membrane works by using the electrochemical process of electrodialysis, which selectively filters out salt from water using a semi-permeable membrane and an electric voltage difference. The team led by Adam Uliana added special nanoparticles to the membrane that react differently to toxic substances depending on their composition and structure. For example, sulfur compounds can filter out mercury atoms from seawater, while other nanoparticles can remove boron, a toxic substance for plants. The experiments also showed that the new membrane is highly efficient and can be reused up to ten times. One kilogram of the material can purify about 35,000 liters of contaminated water.
The researchers plan to develop new materials and methods to remove other problematic substances from water in the future. If successful, this flexible and cost-effective electrodialysis process could significantly improve the drinking water supply in many regions of the world by simultaneously removing salt and toxins. The new membrane technology could be a game-changer in the fight against water scarcity and pollution, providing a sustainable solution for clean water access.