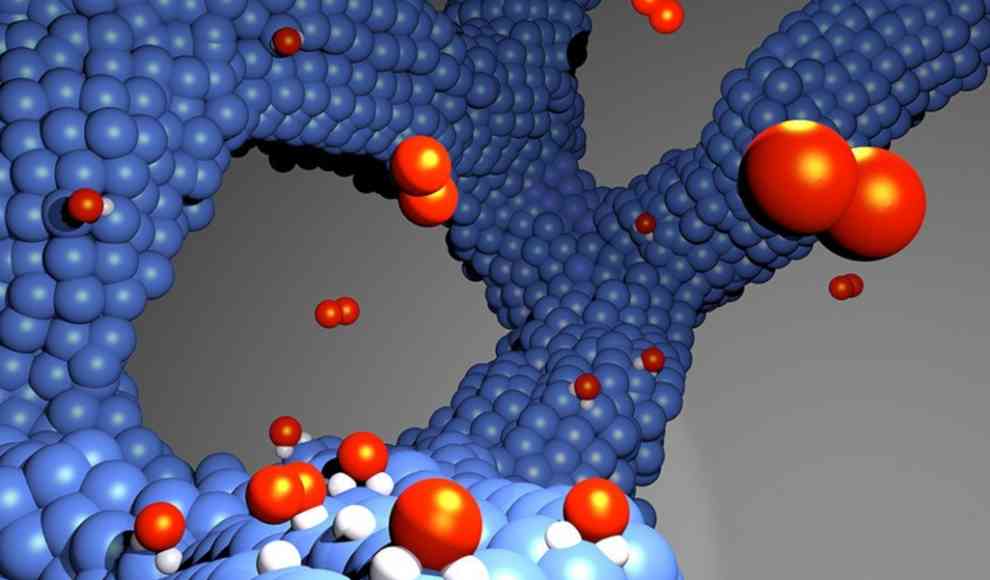
A breakthrough in fuel cell technology has been achieved by scientists at the University of Bern and the University of Copenhagen. They have developed a new catalyst structure that does not require a carbon carrier, which prevents oxidation and maintains the system’s performance over the long term. This technology could be used in electric vehicles, which have a higher range and lower weight than those with battery-powered electric drives. The current catalysts, made of platinum-cobalt particles on a carbon carrier, corrode and lose their efficiency over time, reducing the surface area of the catalyst.
The new catalyst structure is made up of a porous network of catalyst particles, which avoids the corrosion problems of carbon-based catalysts. The structure was created using the cathode sputtering process, which applies gaseous cobalt and platinum atoms in individual layers to a substrate. When condensed in the air, they form a self-supporting, porous network of platinum and cobalt oxide that does not require a carbon carrier. The researchers found that the new catalyst maintained its performance even after 800 charging cycles, losing only 15% of its surface area, compared to a conventional platinum-cobalt catalyst, which lost 53% of its surface area under the same conditions.
The new catalyst promises stable fuel cell operation even at higher temperatures and higher current densities, making it an important development for sustainable energy use, particularly in the transportation sector. The researchers believe that their study results are of significance for the further development of sustainable energy use, especially in the face of current developments in the heavy-duty transportation sector. The technology is industrially scalable and can be used for larger production volumes, such as in the automotive industry.