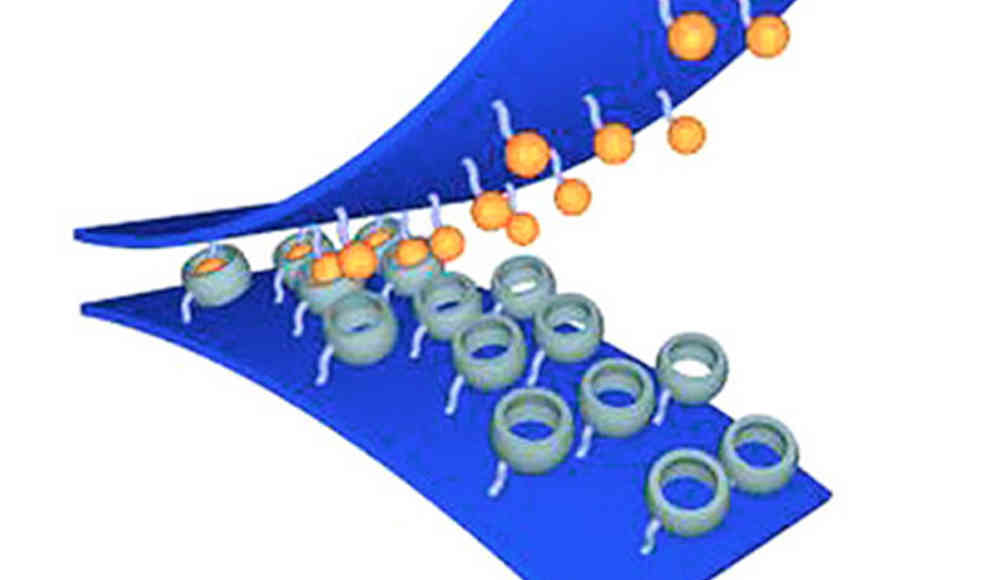
South Korean scientists have developed a new method for reversible underwater adhesion using the water-repellent properties of two molecules that can connect and separate like Velcro. The conventional problem with adhesives that are supposed to stick underwater is the water molecules that move between the adhesive and the surface, preventing a chemical reaction between the two components. Previous research attempted to displace the water molecules between the adhesive and the surface. However, Kimoon Kim and his colleagues from the Pohang University of Science and Technology in South Korea took a different approach in developing a reversible adhesive. They used the necessary displacement of water as a driving force for the bond between the adhesive and the surface.
The researchers created two different silicone strips for the molecular Velcro. On one side, they attached water-soluble host molecules, called cucurbiturils, which consist of several units and can bind other molecules in a water-repellent cavity. On the other side, they attached ferrocenes, which are water-repellent compounds consisting of two rings with an iron atom. Under pressure, the molecules easily slide into the cavities of the other molecules. Because both substances are water-repellent at this point, they can form a very strong but not covalent, i.e., easily reversible, connection with each other. Since the connection is energetically favorable for both sides, the bond promotes the adhesive effect.
The chemical Velcro adhesive is stronger than double-sided tape, as a 1×1 centimeter silicone adhesive tape can carry a weight of two kilograms underwater and four kilograms when dried in the air. To separate the bonded surfaces, a strong pull is sufficient. It is also possible to chemically treat the adhesive with a hypochlorite solution that oxidizes the iron atoms of the ferrocenes and thus releases the adhesion. After separation, the silicone strips, like Velcro, are immediately reusable. The chemical substances used in the adhesive process are all biocompatible, making it possible to use them in medicine, such as closing surgical wounds.