A new gene therapy could replace the current treatment for age-related macular degeneration (AMD), a disease that leads to blindness in seniors. Currently, the only way to slow down the disease is through regular injections. However, scientists at Cornell Medical College in New York have developed a gene therapy that could make the treatment of AMD much simpler. The therapy involves modifying the genes of retinal cells to produce a growth inhibitor against the VEGF growth factor, which causes the disease. The therapy has already been tested successfully on animals and humans, and if further clinical studies confirm its effectiveness, it could be available in three to five years.
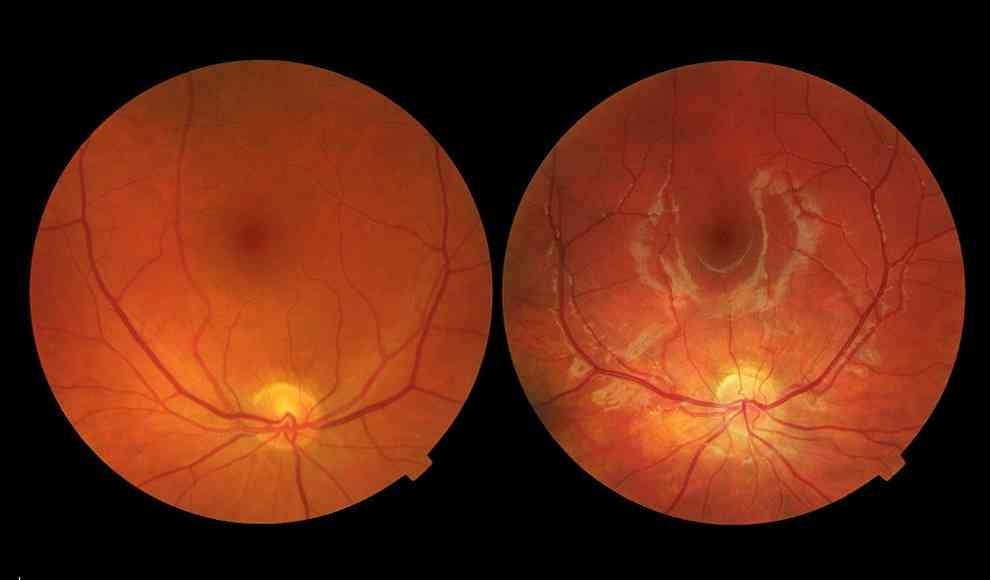
AMD is a disease that destroys the retina, leading to blindness in seniors. There are two types of AMD: the dry variant, which causes pigment cells in the retina to die, and the wet variant, which occurs when fine blood vessels penetrate the eye and retina, destroying eyesight. Currently, there is no known treatment for the dry variant of the disease. The wet variant can be treated with special medications that slow down the growth of the diseased blood vessels. However, patients must receive regular injections directly into the eye, which can be uncomfortable. There is currently no permanent treatment for the disease.
The new gene therapy involves modifying the genes of retinal cells to produce a growth inhibitor against the VEGF growth factor. Scientists at Cornell Medical College in New York have already tested the therapy successfully on animals and humans. The therapy involves injecting DNA into the eye that contains a blueprint for a drug similar to Aflibercept, which is used in conventional therapies. The retinal cells then produce a protein that acts as a growth inhibitor against the diseased blood vessels. The therapy has already been tested on six patients who have not required medication since the therapy was administered six months ago. If further clinical studies confirm its effectiveness, the therapy could be available in three to five years.