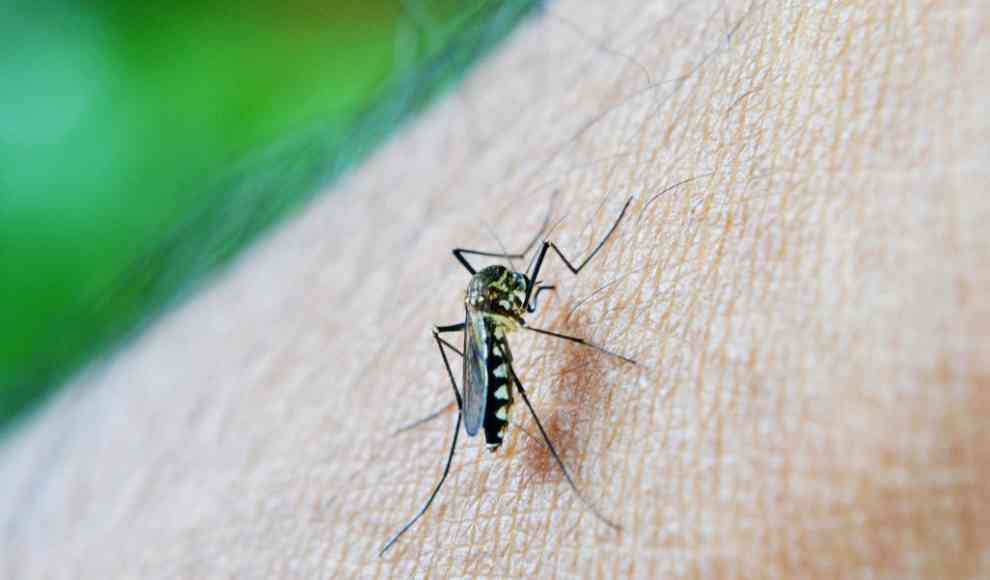
A groundbreaking clinical trial has successfully tested a highly effective malaria vaccine for the first time. Malaria is one of the leading causes of child mortality in Africa, with an estimated 229 million cases and 409,000 deaths in 2019 alone. The disease, transmitted by the female Anopheles mosquito, is responsible for 67% of child deaths in the region. Researchers at the University of Oxford have developed a vaccine called R21/Matrix-M, which exceeded the World Health Organization’s (WHO) target of 75% efficacy by reducing the risk of infection by 77% in a phase-II trial. The vaccine was tested on 450 infants aged five to 17 months in Burkina Faso, who were monitored for a year after vaccination. No serious side effects were reported.
Adrian Hill, director of the Jenner Institute at the University of Oxford, expressed optimism about the vaccine’s potential, stating that “these new results support our high expectations for the potential of this vaccine.” The next step is to conduct a larger phase-III trial in several African countries, involving 4,800 children, in collaboration with US pharmaceutical company Novavax and the Indian Serum Institute. Hill emphasized the need to ensure safety and build on the promising results of the phase-II trial. If the larger trial confirms the vaccine’s high efficacy and low side effects, it could be a crucial breakthrough in achieving the WHO’s goals for World Malaria Day. By 2025, 25 countries, including Guatemala, Honduras, North Korea, and Thailand, are expected to be malaria-free.
This development is significant not only for Africa but for the global fight against malaria, which remains a major public health challenge. The WHO has set ambitious targets for reducing malaria cases and deaths, but progress has been slow. A highly effective vaccine could be a game-changer, especially for vulnerable populations such as young children. The success of the phase-II trial is a promising sign, but more research is needed to ensure that the vaccine is safe and effective for a wider population. The phase-III trial will be a critical test of the vaccine’s potential to save lives and reduce the burden of malaria in Africa and beyond.