A new method of extracting hydrogen from ammonia using LED light, iron, and copper has been developed by scientists at Rice University. Unlike traditional ammonia splitting, this method does not require expensive materials or high heat. Hydrogen can be produced through electrolysis of water using renewable energy sources, but it must be cooled and liquefied or chemically bound for storage and transportation. Liquid ammonia is particularly suitable for this purpose, as it only needs to be cooled to minus 40 degrees Celsius to bind hydrogen. However, a temperature of 400 to 600 degrees Celsius is required to split the liquid ammonia back into hydrogen and nitrogen.
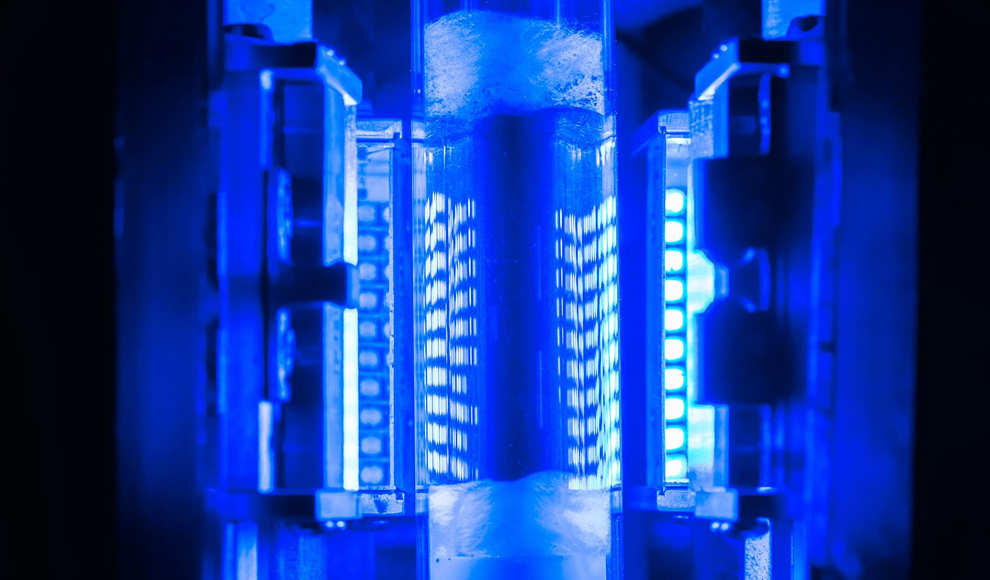
The scientists at Rice University have developed an alternative method using plasmonic photocatalysts to split ammonia. These reaction helpers made of metallic nanoparticles concentrate the energy of incoming light and strongly stimulate electrons in the material. A second metal forms a temporary partner for nitrogen in this process, allowing the bound hydrogen to be separated. The researchers created a photocatalyst with iron as a chemical splitting agent and copper as a light antenna. They then tested the splitting process using laser pulses and found that the copper-iron catalyst showed reactivity and efficiency comparable to that of the commonly used copper-ruthenium catalyst.
The researchers then tested whether the splitting could be done on an industrial scale without lasers. They conducted an experiment in a 500-times larger light reactor using LEDs with a wavelength of 470 nanometers. During the experiment, ammonia was continuously passed through two reaction tubes past the photocatalyst. The researchers found that the copper-iron catalyst showed a very high photolytic reactivity under LED lighting, with a 72% conversion of ammonia and a yield of 14 grams of hydrogen per day. While the process currently requires more energy than traditional ammonia crackers, the researchers believe that optimization and scaling of the process could reduce energy consumption to just a few kilowatt-hours per kilogram of hydrogen. This method could be used for cheap and decentralized ammonia splitting, making hydrogen a more affordable and clean fuel with high availability.