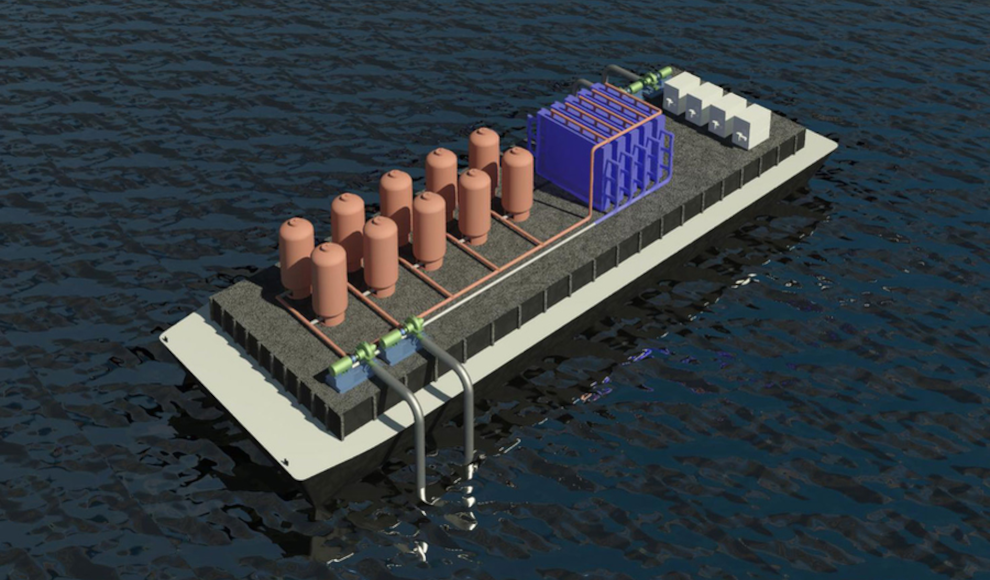
The world’s oceans absorb a significant amount of carbon dioxide from the atmosphere, causing them to become increasingly acidic. This process harms marine life, particularly coral reefs. However, researchers at the Massachusetts Institute of Technology (MIT) have developed a new technique to efficiently remove CO2 from seawater. The system consists of two chambers that are filled with seawater. In the first chamber, reactive electrodes release protons into the water, which acidifies it and converts the inorganic bicarbonates into molecular CO2. The gas is then collected, and the water is directed to the second chamber, where the electrodes have a reversed voltage. This process removes the protons from the water, converting the acidic water back into alkaline water that can be returned to the ocean.
According to the researchers, the system could be combined with existing facilities, such as seawater desalination plants or water treatment systems on ships. This could help offset emissions from shipping companies. Additionally, the captured CO2 could be used as a raw material for the production of synthetic fuels and ethanol. However, the researchers caution that not all of the captured CO2 can be used for this purpose, and a significant portion would need to be stored. The team plans to build a demonstration plant for the new technology within the next two years.
This breakthrough could have significant implications for the health of our oceans and the fight against climate change. By removing CO2 from seawater, we can reduce the acidity of the oceans and protect marine life. Additionally, the captured CO2 could be used to produce sustainable fuels, reducing our reliance on fossil fuels. The MIT researchers have shown that innovation and technology can play a crucial role in addressing the challenges of our time.