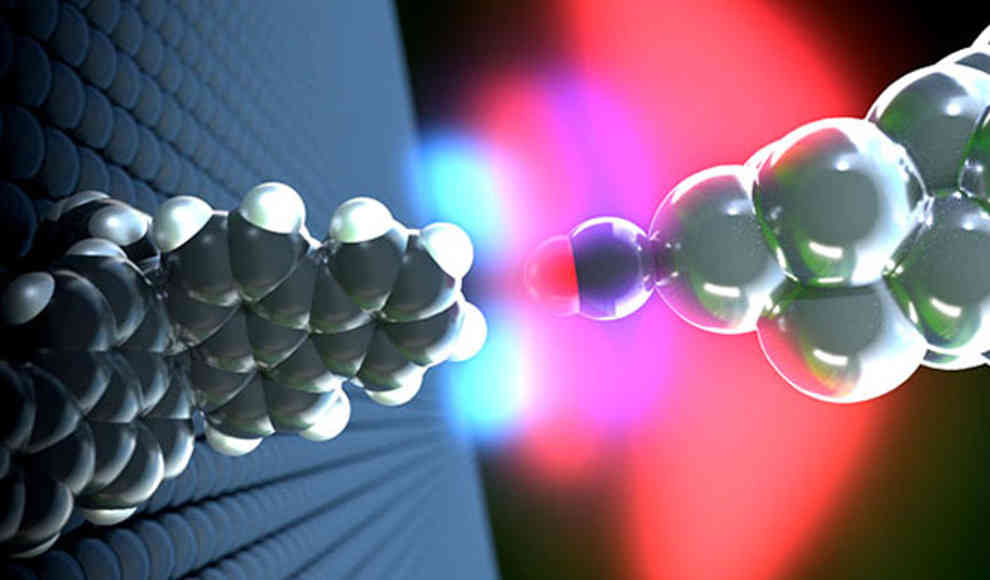
Scientists have made a groundbreaking discovery by using a scanning probe microscope to observe the formation of a hydrogen bond in a single molecule. The researchers reported in the journal Science Advances that this method could be used to identify complex organic molecules in the future. Hydrogen is the most abundant element in the universe, and hydrogen bonds are responsible for the structure and function of DNA, proteins, and polysaccharides. The formation of a hydrogen bond occurs when the positively charged hydrogen atoms of a water molecule or another molecule come into contact with negatively charged atoms such as the oxygen atom of a water molecule. The atoms are attracted to each other, similar to two magnets with different poles.
Previously, researchers had been unable to observe the formation of a hydrogen bond and had not obtained clear results with a scanning microscope. However, a high-resolution scanning microscope allowed Dr. Shigeki Kawai and his team from the Swiss Nanoscience Institute and the University of Basel to analyze hydrogen atoms in a single hydrocarbon compound. The microscope’s unique feature is a probe arm with a very small tip at the bottom. The scientists froze a single carbon monoxide molecule to the tip, which was cooled to about minus 268 degrees Celsius, and the downward-pointing oxygen atom of the molecule became the end of the tip. The researchers chose a propeller-like molecule for their experiment, which they attached to their slide. The propellanes on the surface were arranged so that two hydrogen atoms always pointed towards the probe arm. When Kawai and his colleagues brought the carbon monoxide-tipped probe of the scanning microscope near the two hydrogen atoms, they observed the formation of hydrogen bonds, which could be analyzed.
The scientists explained that hydrogen bonds are much weaker than chemical bonds but significantly stronger than intermolecular van der Waals forces. The forces and distances between the oxygen atom of the CO molecule on the microscope tip and the hydrogen atoms of the propellane observed in the experiment matched the calculations of Finnish research partners, confirming that the bond was undoubtedly a hydrogen bond and ruling out the much weaker van der Waals forces and stronger ionic bonds. According to the researchers at the Swiss Nanoscience Institute at the University of Basel, this technique could pave the way for identifying complex molecules such as DNA or polymers in the future.