In a groundbreaking move, the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK has approved the use of Casgevy, a gene-editing drug based on the CRISPR/Cas system. This revolutionary therapy can help treat two blood disorders and is authorized for use in patients aged 12 and above. Clinical trials have shown no adverse side effects. The CRISPR/Cas system is a powerful tool that can cut, modify, and insert individual genes. It is considered one of the most significant discoveries in recent years, with Emmanuelle Charpentier and Jennifer Doudna receiving the Nobel Prize in Chemistry in 2020 for their work on it.
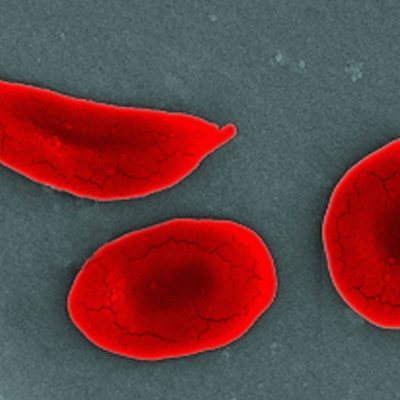
The first human to receive treatment with the CRISPR/Cas system for hereditary transthyretin amyloidosis (hATTR) was in 2021 as part of a clinical phase-1 study. Now, the MHRA has become the first regulatory agency in the world to approve a drug based on this system. Casgevy can be used to treat beta-thalassemia and sickle cell anemia, both of which are painful, lifelong conditions that can be fatal in some cases. These diseases are caused by errors in the genes for hemoglobin, which red blood cells need to transport oxygen through the body.
Clinical studies have shown that Casgevy can restore adequate hemoglobin production in most patients with these blood disorders, significantly reducing their symptoms. The therapy involves taking stem cells from the patient’s bone marrow, modifying the faulty gene in the lab so that it can produce hemoglobin in the body, and then infusing these edited cells back into the patient. The MHRA has reported no significant safety concerns or side effects from the trials.
Samarth Kulkarni, CEO of Vertex Pharmaceuticals and Crispr Therapeutics, has praised the approval of Casgevy, expressing hope that it will be the first of many applications of this Nobel Prize-winning technology to benefit patients with serious illnesses. This breakthrough in gene-editing therapy could pave the way for more treatments for a range of genetic disorders, offering hope to millions of people worldwide.